4 de April de 2022
Evolution and characteristics of quaternary ammoniums for surface disinfection
Cleaning and disinfection of surfaces and environments in contact with food are essential in the food industry and catering to maintain a degree of hygiene in accordance with the established requirements. To this end, as an essential part of the implementation of a HACCP system, it is essential to develop, implement and execute cleaning and disinfection protocols adapted to the needs of each facility, the objective of which is to prevent physical-chemical and/or microbiological contamination, in order to guarantee the safety of the food produced. In this way, it is possible to avoid cases of food poisoning, extend the shelf life of food and prevent organoleptic changes.
The design phase of the cleaning and disinfection procedures plays a transcendental role in achieving the above objectives. The protocols established must be well detailed, easy to implement and monitor, and ensure that the objectives set are achieved.

Cleaning and disinfection protocols
One of the main aspects to be considered during the design phase is the choice of the products used for the cleaning and disinfection protocols, and in particular the disinfectant products. We currently have a wide range of biocidal active ingredients regulated by the Biocides Regulation (EU) 528/2012 (chlorine, quaternary ammoniums, alcohols, glutarladehyde, peroxides and peracids, amines, etc.). Each of them has certain advantages and disadvantages that must be taken into account when choosing the cleaning and disinfection protocols.
Introduction to quaternary ammoniums
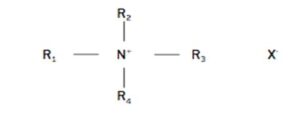
Quaternary ammoniums are chemical compounds classified within the group of cationic surfactants. Its general structure comprises a cationic portion composed of a nitrogen atom attached to four alkyl chains (functional part of the molecule) and a halogen atom (usually chlorine).
They were developed in 1916 by Jacobs and Heidelberg[1] who already highlighted their biocidal properties, and were improved in 1935 by Domagk[2], who proposed that the attachment of an aliphatic group to the quaternary nitrogen improved the biocidal properties of the compound. Domagk developed alkyl dimethyl benzyl ammonium chloride (ADBAC) which is considered to be the first generation quaternary ammonium.
Subsequently, the substitution of hydrogen in the aliphatic ring for an ethyl group gave rise to the second generation of quaternary ammoniums. Known as ADEBAC, alkyl dimethyl ethylbenzyl ammonium chloride.
In 1955 the third generation of quaternary ammoniums was created with the combination of ADBAC and ADEBAC, which provided improvements in their biocidal activity and detergency while decreasing their toxicity.
Technical improvements in chemical synthesis allowed the fourth generation of quaternary ammoniums to be developed in 1965. Namely alkyl dimethyl ammonium chloride (DDAC) which is characterised by a higher biocidal efficacy compared to previous generations, especially evident in conditions of presence of organic dirt and/or hard water.
The fifth generation of quaternary ammoniums comprises mixtures in different proportions of DDAC and ADBAC to obtain a wide range of action against the maximum number of micro-organisms.
| Generation | Compound(s) |
| FIRST | ADBAC |
| SECOND | ADEBAC |
| THIRD | ADBAC + ADEBAC |
| FOURTH | DDAC |
| FIFTH | DDAC + ADBAC |
Characteristics of quaternary ammoniums
Their neutral nature and relative harmlessness make quaternary ammoniums an ideal compound for the disinfection of surfaces and environments. Among its main advantages we find:
- Broad bactericidal, fungicidal and virucidal spectrum. Its mechanism of action, penetrating and breaking the cytoplasmic membrane, degrading proteins and nucleic acids and finally causing cell lysis, gives it excellent properties against all types of micro-organisms.
- Low corrosiveness. They do not attack most industrial and institutional surfaces and their handling is relatively safe compared to that of other disinfectant ingredients, provided the pertinent protective measures are used.
- Effectiveness even in the presence of organic matter, especially in the case of the latest generations of quaternary ammoniums.
- Residual power. Their physical-chemical characteristics ensure that, if not rinsed, they remain on surfaces and retain their disinfectant efficacy for a long time.
Some of the disadvantages they may have are as follows:
- Incompatibility with anionic surfactants, which makes it difficult to formulate together with some detergents.
- Low effectiveness against spore-forming microorganisms, being considered sporostatic rather than sporocidal compounds[3]. The wall of sporulates when in vegetative form is not very permeable to quaternary compounds, which hinders their mechanism of action.
- Difficult to apply in processes using pumps and recirculation systems, due to its tendency to foam.
- Its substantivity on surfaces makes it more difficult to rinse than other disinfectants. Current regulations require that surfaces that will come into contact with food must be rinsed to ensure that there are no traces of chemical disinfectants that could contaminate the food:
- At the European level, the general guidelines for determining the Maximum Residue Limit (MRL) in food are determined by Regulation (EU) 396/2005. For quaternary ammoniums, the limits set by Regulation (EU) 1119/2014 are 0.1 mg/kg of food (0.01 mg/kg for infant formulae and milk powder). There are proposals developed by the ECHA working group for establishing the transfer of residues to food[4] which, once the calculations have been adapted, allow us to establish values of <0.5mg/m2 on surfaces and <0.1mg/L in rinsing water as limit values equivalent to the MRLs in food for these biocidal active ingredients. These values are easily achieved by adequate rinsing with water after application of the disinfectant product.
- According to FDA regulations (Food Code 2009: Chapter 7. Poisonous or Toxic Materials - subpart 204.11 -) sanitising products and other chemical disinfectants applied to food-contact surfaces must meet the requirements specified in the CFR regulation (40 CFR 180.940 Tolerance exemptions for active and inert ingredients for use antimicrobial formulations - food-contact surface sanitising solutions -). Quaternary ammoniums are included in this list, without the need for rinsing, but guaranteeing adequate drainage, in maximum concentrations ranging from 200 to 400 ppm depending on the quaternary compounds used.
- Possibility of causing resistance phenomena. Although uncommon, incorrect use or working at sub-lethal concentrations can lead to the emergence of tolerant micro-organisms that make it necessary to increase the dose and/or combine different types of biocides[5].
Conclusions: quaternary ammoniums, disinfectants for food surfaces
The wide range of advantages provided by quaternary ammonium compounds make them one of the first choices when designing Cleaning and Disinfection protocols and choosing a disinfectant product for food, veterinary or institutional surfaces (PT4, PT3 and PT2 biocides, respectively, within the framework of the Biocides Regulation (EU) 528/2012).
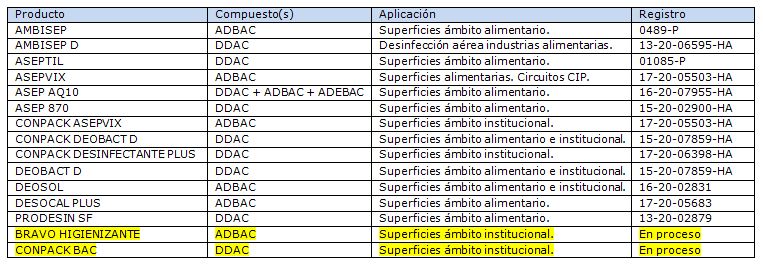
In order to respond to all these applications, PROQUIMIA has a wide range of disinfectant products based on quaternary ammoniums:
Bibliographic references:
[1] W. A. Jacobs, 1916. The Bactericidal properties of the quaternary salts of hexamethyltetramine. I. The problem of the chemotherapy of experimental bacterial infections. J. Exp. Med., 23: 563-568.
[2] G. Domagk, 1935. Eine neue Klasse von Disinfektionsmitteln. Dtsch. Med. Wochschr., 61: 828-832.
[3] Russell A D. Mechanisms of bacterial resistance to biocides. Int Biodeterior Biodegrad. 1995.
[5] S. Langsrud G. Sundheim R. Borgmann‐Strahsen, 2003. Intrinsic and acquired resistance to quaternary ammonium compounds in food‐related Pseudomonas spp.
AUTHOR: Dani Calvente
Do you want more information?
We help you
In accordance with Regulation 2016/679 (GDPR) the basic information on personal data protection is provided below:
- Data controller: PROQUIMIA, S.A.
- Purpose of processing: Managing the sending of information, resolving queries and/or collecting data for possible business relationships.
- Legal Basis: Consent of the person concerned
- Recipients: No data will be transferred to third parties, unless this is legally obliged.
- Rights: Access, rectification, deletion, opposition, limitation, portability and presentation of claims.
- Additional information: Additional and detailed information on Data Protection can be found on our website: Privacy policy